November 06, 2015
Insanely high drug prices have been in the news lately. We are regularly hearing about new miracle drugs like the Hepatitis C drug Sovaldi. Sovaldi comes with an $84,000 price tag for a 3-month course of treatment. Many of the new cancer drugs cost well over $100,000 for a year’s dosage. And of course we had the case of Turing Pharmaceuticals, which raised the price of a Daraprim, an old but important anti-infection drug, by 5000 percent.
These stories of extraordinarily high drug prices are especially painful because they are unnecessary. In almost all cases drugs are cheap to produce. The reason they are expensive is because the government grants them a patent monopoly. (In the case of Daraprim, at the moment Turing is the only licensed manufacturer, even though the drug is off-patent.) Generic Sovaldi is available for just $300 a treatment in Egypt, less than one percent of the U.S. price. Most of the cutting edge cancer drugs would also be available for less than one percent of the U.S. price if they could be sold as generics in a free market.
The rationale for patent monopolies is that the drug companies need high prices to recover their research costs. And, they claim they have very high research costs. According to Joe DiMasi, an economist with close ties to the industry, the research and development costs of the pharmaceutical industry averages almost $2.6 billion for each new drug they produce that is a new molecular entity. (New molecular entities account for only about 15 percent of the new drugs approved by the Food and Drug Administration.)
Patent monopolies are not the only way to support research. There are other mechanisms. For example, the U.S. government spends over $30 billion a year on biomedical research through the National Institutes of Health. There are also various private initiatives that support research.
One such initiative is the Drugs for Neglected Diseases Initiative (DNDI). This is a research network, led by Doctors Without Borders, that was established to develop treatments for diseases that primarily affect poor people in the developing world. It was created in 2002. On their tenth anniversary, DNDI produced a report describing some of their accomplishments. The figure below shows some of the highlights and their price tag and compares them to DiMasi’s estimate of what it costs the big pharmaceutical companies to develop a single drug.
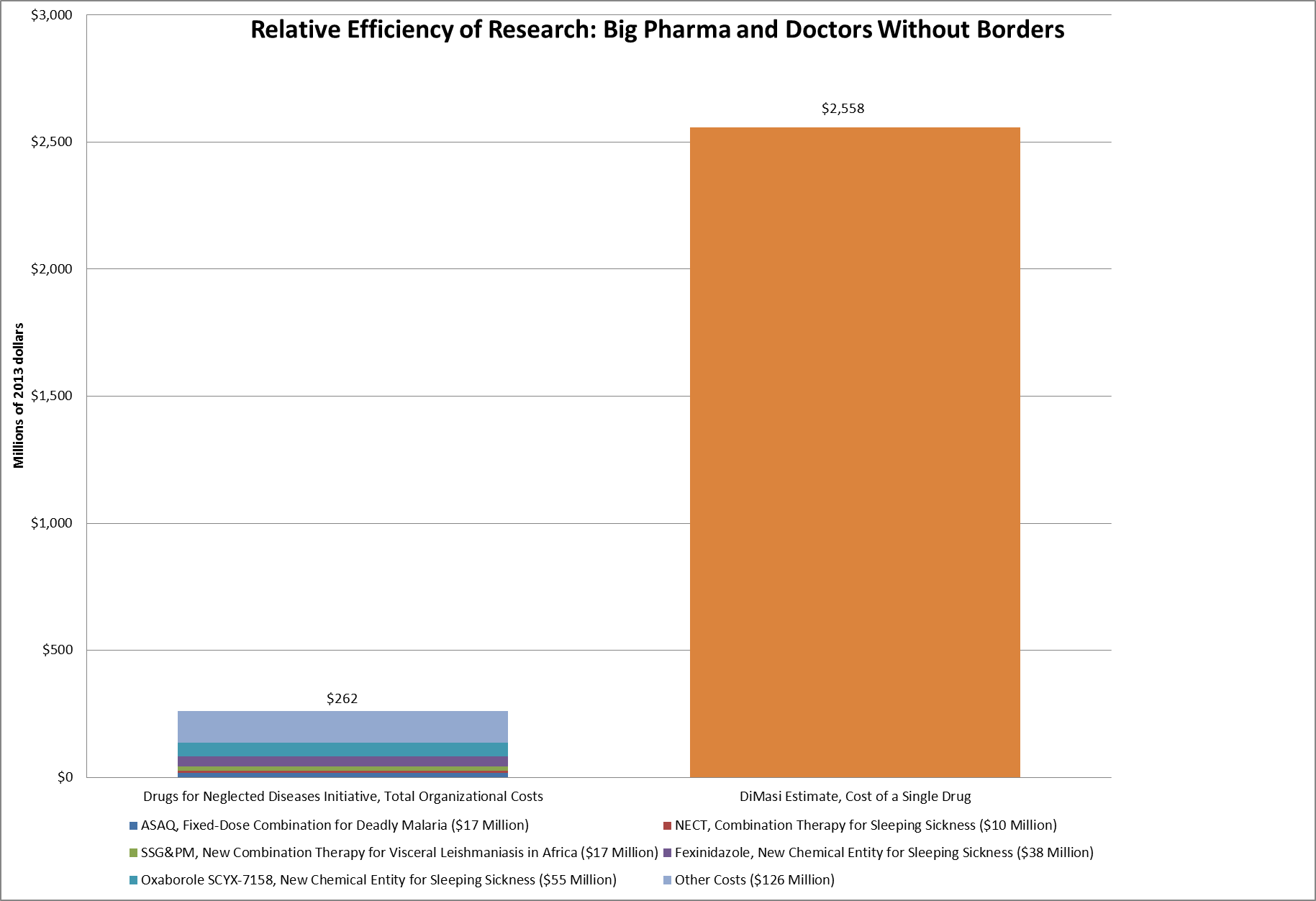
Source: DNDI and DiMasi, 2014.
As the figure shows, DNDI was able to develop ASAQ, a combination drug for treating Malaria, for $17 million. More than 250 million dosages have been distributed since 2007. It developed Fexinidazole, a new drug candidate and new chemical entity, intended to treat sleeping sickness, at a cost of $38 million. DNDI developed SSG&PM, a combination therapy for visceral leishmaniasis at a cost of $17 million. DNDI’s entire budget for its first 10 years of existence was $242 million, less than one-tenth of what DiMasi estimates it costs the pharmaceutical industry to develop a single new drug.
This is not an entirely apples to apples comparison. The DNDI spending figures do not include any imputation for interest costs. Using DiMasi’s methodology this could come close to doubling its expenditures. In addition, DNDI gets many donations in kind, the value of which are not included in these calculations.
Nonetheless, it is hard to escape the conclusion that DNDI research is far more efficient than patent supported research by the pharmaceutical industry. And no one has to struggle to come up with tens or hundreds of thousands of dollars to buy the drugs developed by DNDI. They are all available as low-cost generics.
This massive discrepancy in costs should generate some interest in emulating the DNDI model for other drugs. At the very least it would be desirable to have the clinical trial portion of the testing publicly funded, with all approved drugs available as generics from the beginning. (The companies conducting the tests could buy up all rights to a drug before they begin the testing.)
The tests themselves could be fully public, with the baseline characteristics of the treatment and control group, along with their progress in the treatment, placed on the web. This means that we would know whether women did better with a drug than men, if the young did better than the old or whether there were complications if people with other health conditions took the drug. Such disclosure would allow other researchers to benefit from the information in their own work and for doctors to assess the results in determining which drugs are best for their patients.
Publicly funded trials would also eliminate the incentive and ability of drug companies to misrepresent research findings that reflect poorly on their drug. Drug companies have caused enormous harm by exaggerating the effectiveness of their drugs and concealing evidence of harmful effects. If the company doing the testing has no special stake in having large numbers of people take their drugs, they would have no incentive to not be truthful about their results.
The Obama administration is planning a forum on high drug prices later this month. It would be unfortunate if the forum didn’t discuss new mechanisms for financing drug research. And, publicly funded clinical trials should be the top item on the list of new mechanisms.
Addendum:
I’ve learned a lot from reading the comments to this post. I realize that many people thought I was making an apples to apples comparison in looking at the costs incurred by DNDI and the pharmaceutical industry. Undoubtedly they were confused by the part where I wrote:
“This is not an entirely apples to apples comparison. The DNDI spending figures do not include any imputation for interest costs. Using DiMasi’s methodology this could come close to doubling its expenditures. In addition, DNDI gets many donations in kind, the value of which are not included in these calculations.”
And, I see hibob seems to believe he is vindicating the pharmaceutical industry by pointing out how much money it spends on research. Actually, the point is not to spend lots of money on research, nor is it even to develop new chemical entities. The point is to improve people’s health, and it looks like the current patent system is an incredibly inefficient mechanism for bringing about that income. Telling us that the industry spends tons of money on research is an argument for its inefficiency, not against it.





Comments